Biologics, for the longest time, had been enjoying freedom from generic erosion. Now, biosimilars are an impending threat to these biologics.
FDA approval of a biosimilar is only the first hit. Upon FDA approval, payers are already using publicly available data and approved labeling to see how they can leverage biosimilars in their contracting strategies.
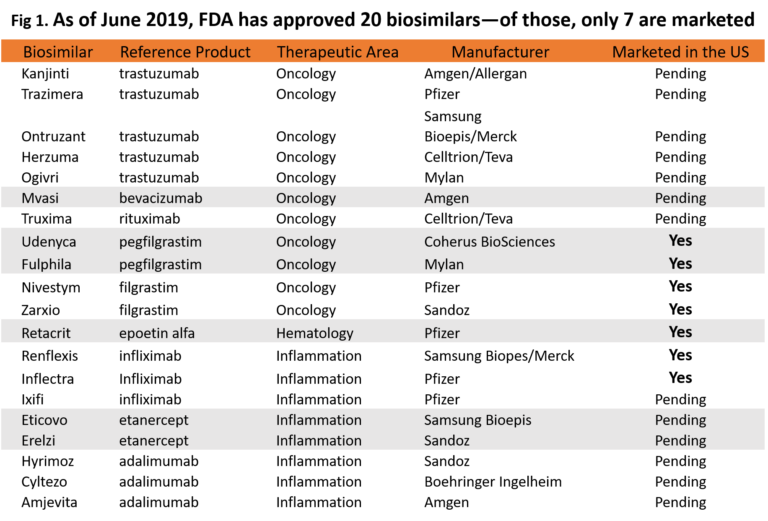
Even so, the LAUNCH of the biosimilars is what really puts a dent in the revenue. Biosimilar launch is an important milestone, considering only 7 of the 20 currently FDA approved biosimilars have launched into the market (see Figure 1 below for my summary of approved and marketed biosimilars in the US). Threat of patent litigation is the biggest hurdle to overcome for biosimilars before they can launch into the market.
Despite biosimilar launch, the reference product might be able to retain a reasonable market share—UNLESS, the biosimilar is designated as interchangeable by the FDA. The FDA recently issued the final guidance on interchangeability designation last month. I believe that biosimilars deemed as interchangeable by the FDA can cause a lethal blow to the reference product (in terms of market access and subsequent market share), because that’s when a biosimilar can truly behave like a generic drug and cause that “generic erosion” that we all know about.
So, what are the options for manufacturers of the reference products?
A) Take cover, you’re in for a hit
B) Start a campaign to downplay the importance of biosimilars
C) Innovate to bring a meaningful change to the market
When in doubt, go with ‘C.’ The adage didn’t always serve me well in pharmacy school, but that IS the correct answer here (former commissioner of the FDA, Scott Gottlieb, would agree with me).
The biosimilar threat is real. However, having a good pulse on the market will enable a sound strategy to retain—and perhaps even grow—the market share of the reference product.
According to an insightful analysis by Rand, Amgen has been able to successfully put up a fight against not just 1, not 2, not 3, but 4 biosimilars in the market through innovation.
Genentech’s Herceptin (trastuzumab) is up for a similar fight against an army of 5 biosimilars (already approved, though yet to launch). Innovation seems to be the name of the game for Genentech as well: interestingly, in February 2019, FDA approved Genentech’s Herceptin HYLECTA, a subcutaneous version of the intravenous Herceptin. If Genentech can successfully serve this innovation up to payers and organized customers, such as IDNs, with the proper outcomes data, I think Genentech will be just fine.
Innovation is certainly a steep climb, and Amgen and Genentech are clearly taking the high road to accept this challenge.
This just goes to show that there is ALWAYS room for improvement. Furthermore, those who are agents of change that continue to improve, take the cake. In the famous words of Steve Jobs, “Innovation distinguishes between a leader and a follower.”
It’s time to make big strides and turn heads. Ready. Set. Access.

